Fats produced by microbes could provide a source of renewable biofuels and other products currently made from petroleum
The Science
The cell envelope plays an important role in the life cycle of bacteria, controlling its shape and creating a physical barrier to protect it from the environment. Bacteria must manage cell growth and division to survive and reproduce. Disrupting that process leads to release of fatty acids, or lipids. Two new studies shed light on the process in one, and likely other, species. One study identifies a previously unknown protein responsible for cell envelope integrity. The other reports on a system that regulates envelope functions that can be altered to increase lipid production.
The Impact
Fats produced by microbes could provide a source of renewable alternatives to petroleum-derived compounds like lubricants and jet fuel. Some microbes produce lipids naturally, but many only do so when starved of nutrients. One way to increase production is to genetically rewire the circuits that limit lipid production. In 2017, GLBRC scientists found two such mutant strains that produced high levels of lipids by squeezing them out of their cell envelope, making the oil easier to harvest. These new studies help explain the mechanisms these bacteria use to shed their fat and reveal new ways to increase lipid secretion.
Summary
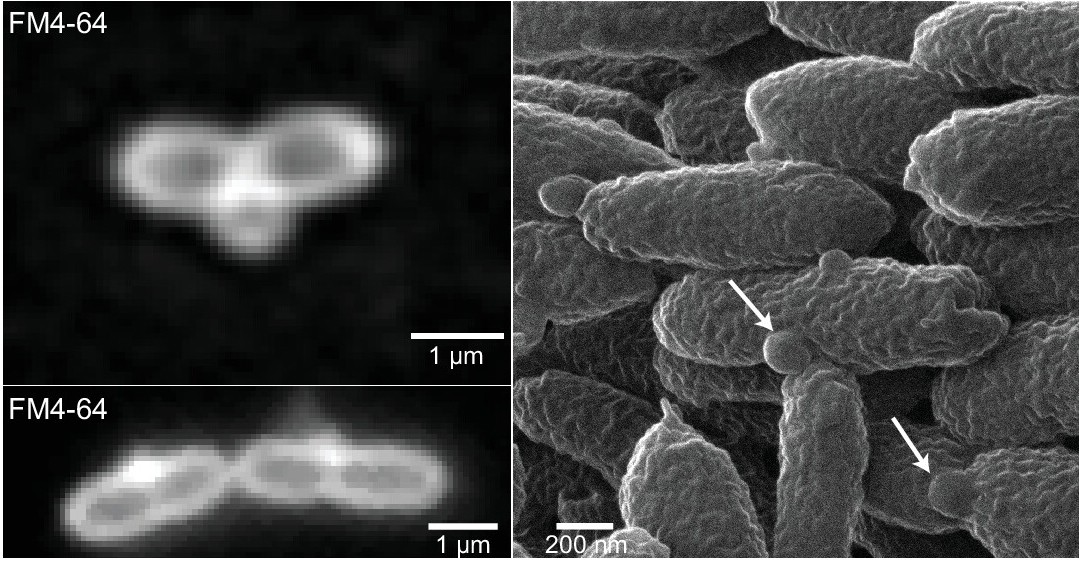
Scientists with the Great Lakes Bioenergy Research Center analyzed two mutants of the Gram-negative bacterium Rhodobacter sphaeroides that secrete lipids.
One study identifies a two-component regulatory system called CenKR, which controls the genes involved in maintaining the integrity of the cell envelope. They found that increasing the CenKR activity caused cells to become longer and stick together because of loosening of the mesh-like structure that forms the cell wall, which were revealed with a cryo-electron microscope and three-dimensional imaging techniques.
The other study identified a previously uncharacterized envelope protein controlled by the CenKR regulator. They propose that this protein, called DalA (for Division Associated Lipoprotein A), serves as a scaffold that allows other enzymes to build the mesh needed for cell division and envelope integrity. In addition, they used high-resolution cell imaging to show that a DalA mutant secretes materials from the cell division site.