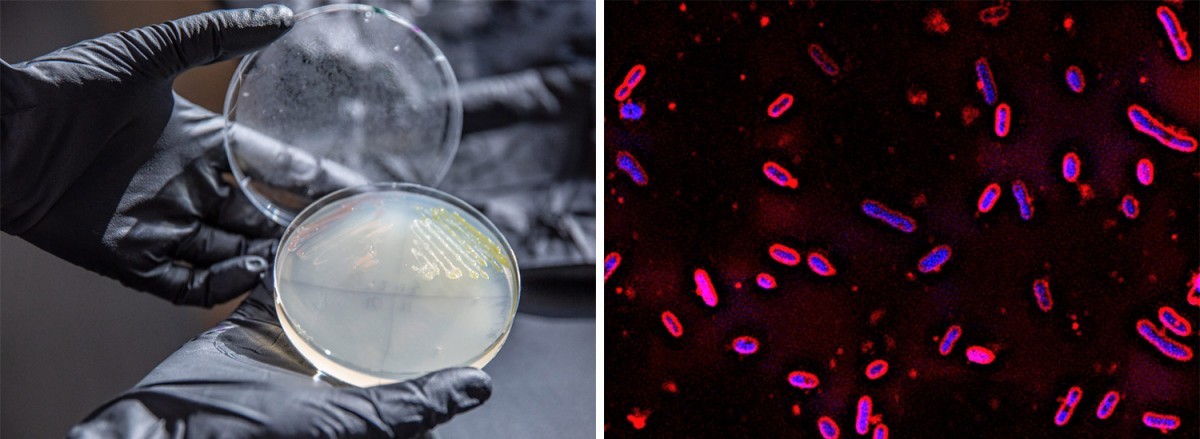
Engineered strains of Novosphingobium aromaticivorans convert abundant but underutilized biomass into valuable replacements for petrochemicals.
The Science
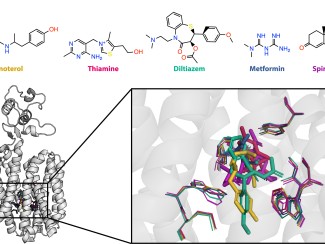
Muconic acid is a chemical building block that can be used to make plastics such as those used in food and drink packaging, which are typically made from fossil fuels. Many bacteria produce this compound as a step in the process their cells use to capture energy. But while these microbes can make muconic acid from food-grade sugars, they either lack or have limited ability to process the mix of aromatic compounds in another major part of the plant cell called lignin. Novosphingobium aromaticivorans is a soil microbe that can eat a wide range of aromatic compounds. Here, University of Wisconsin–Madison scientists genetically modified strains that produce muconic acid from this underused form of biomass.
The Impact
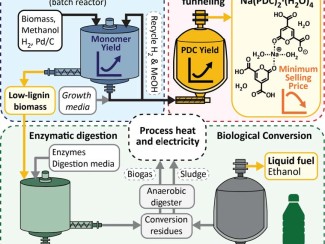
Lignin is the world’s largest renewable source of aromatic carbon, but the complex and irregular structure makes it hard to break down into useful components, and most industrial microbes cannot process the mix of aromatics in lignin that has been chemically separated from plant sugars. Converting this abundant raw material into valuable products is one of the keys to making plant-based products a cost-competitive alternative to fossil fuels and petrochemicals, the primary drivers of climate change.
Summary
Scientists with the Great Lakes Bioenergy Research Center identified genes in the Novosphingobium aromaticivorans bacterium that encode previously undocumented enzymes required to convert aromatic intermediates to cis,cis-muconic acid (ccMA), which they confirmed with lab experiments. Next, they engineered two strains, one using the bacterium’s own genes and one using genes from other bacteria known to produce ccMA. Both engineered strains fully converted the aromatic compounds in liquor derived from alkaline pretreated poplar biomass to ccMA. The findings expand the knowledge of aromatic catabolic pathways in N. aromaticivorans and provide a host microbe that can be used for future studies to optimize ccMA production in bioreactors.
Previous GLBRC research has identified genetic modifications that turn N. aromaticivorans into multitasking microbes able to simultaneously convert lignin aromatics into another plastic building block, 2-pyrone-4,6-dicarboxylic acid, and carotenoids, a group of organic pigments used in nutritional supplements, drugs, and cosmetics.