UW–Madison scientists engineer bacteria to make two valuable products from plant fiber
We often look to the smallest lifeforms for help solving the biggest problems: Microbes help make foods and beverages, cure diseases, treat waste, and even clean up pollution. Yeast and bacteria can also convert plant sugars into biofuels and chemicals traditionally derived from fossil fuels, which are a key component of most plans to slow climate change.
Now University of Wisconsin–Madison researchers have engineered bacteria that can produce two products – at the same time – from underutilized plant fiber. And unlike humans, these multitasking microbes can do both things equally well.

“To my knowledge, it’s one of the first times you can make two valuable products simultaneously in one microbe,” said Tim Donohue, UW–Madison professor of bacteriology and director of the Great Lakes Bioenergy Research Center.
The discovery, detailed in a paper in the December issue of the journal Applied and Environmental Microbiology, could help make those biofuels more sustainable and commercially viable by tapping into multiple chemical markets.
“In principle, the strategy lowers the net greenhouse gas emissions and improves the economics,” Donohue said. “The amount of energy and greenhouse gas that you need to make two products in one pot is going to be less than running two pots to make one product in each pot.”

Every molecule counts
The quest to replace fossil fuels with sustainable alternatives hinges on extracting the most possible value from renewable biomass. Just as with petrochemicals, every molecule counts: Low-volume, high-value products help keep the fuel more affordable.
One of the biggest barriers is a part of the plant cell wall called lignin. Lignin is the most abundant terrestrial source of renewable aromatic carbons, but its irregular structure makes it notoriously difficult to break apart into useful components.
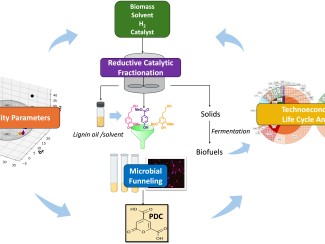
That's why scientists with GLBRC have studied a bacterium named Novosphingobium aromaticivorans (sometimes referred to as simply Novo), which can digest many components of lignin and is relatively easy to genetically modify.
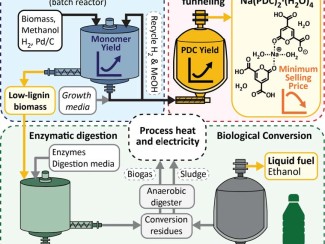
In 2019, researchers engineered a strain of Novo that can produce a key ingredient of plastics like nylon and polyurethane known as PDC. More recently, a team in Donohue’s lab discovered another modification that allows Novo to make a different plastic ingredient called ccMA.
But they didn’t stop there.
“We’re not going to solve our carbon emissions problem by only producing two products,” said Ben Hall, a recent doctoral graduate and co-author of the paper.

Donohue’s team used genomic modeling to come up with a list of potential products that could be made from biomass aromatics. Near the top of the list was zeaxanthin, one of a group of organic pigments known as carotenoids.
Carotenoids, which give carrots, pumpkins, salmon and even flamingos their distinctive hues, are used as nutritional supplements, pharmaceuticals, and cosmetics and have a cumulative market value worth tens of billions of dollars a year.
Researchers knew that Novo had the genes to produce another carotenoid with little market value. Based on the bacteria’s genome sequence, they suspected zeaxanthin is a stepping stone to that less valuable carotenoid in the process that cells use to make complex molecules. It was just a matter of altering the right genes to stop the digestive assembly line at the more valuable product.
By deleting or adding selected genes, they engineered strains that produced zeaxanthin as well as other valuable carotenoids — beta-carotene, lycopene and astaxanthin — when grown on an aromatic compound commonly found in lignin.
Next, the team showed that the engineered bacteria could produce the same carotenoids from a liquor made from ground and treated sorghum stems, a solution that contains a mixture of aromatics that many industrial bacteria can’t digest.

One pot, two products
Hall then wondered what would happen if he combined the genetic changes needed to make PDC and a carotenoid in the same microbe.
The resulting strains produced both PDC and the target carotenoid – with no discernable loss to either yield. Even better, the bacteria accumulate carotenoids within their cells, which must be separated from the solution that contains the PDC, which they excrete.
“We’re already separating the cells from the media,” Hall said. “Now we would have a product coming out of both.”
The next steps include testing whether engineered strains can co-produce carotenoids and ccMA, which Donohue thinks is possible, and to engineer strains to improve yields in industrial conditions.
While there are lucrative markets for each of these products, Donohue and Hall say the real value of the discovery is the ability to add multiple functions to this biological platform.
“To me, it's both the strategy and the products,” Donohue said. “Now that we've done this, I think it opens the door to see if we can create other microbial chassis that make two products.”