Scientists use molecular dynamics simulation to understand how solvents interact with lignin and catalysts for better conversion into valuable chemicals.
The Science
Plant cell walls are made of three main polymers, or long chains of molecules: cellulose, hemicellulose, and lignin. Cellulose and hemicellulose are sugars that can be converted into biofuels. Lignin is a glue-like polymer that binds them together and makes the plant sturdy. Lignin contains ring-shaped molecules called aromatics that can be made into fuels, plastics, and other products. The challenge is breaking apart these molecules, which are linked through strong and unpredictable bonds.
One approach uses a solvent, such as alcohol or water, together with a metal catalyst, a substance that speeds up chemical reactions without being consumed. This process, called reductive catalytic fractionation (RCF), can produce high yields of aromatic building blocks. The choice of catalyst and solvent can affect the types and amounts of products from RCF, but scientists have limited understanding of how solvent selection affects lignin interactions at the molecular level.
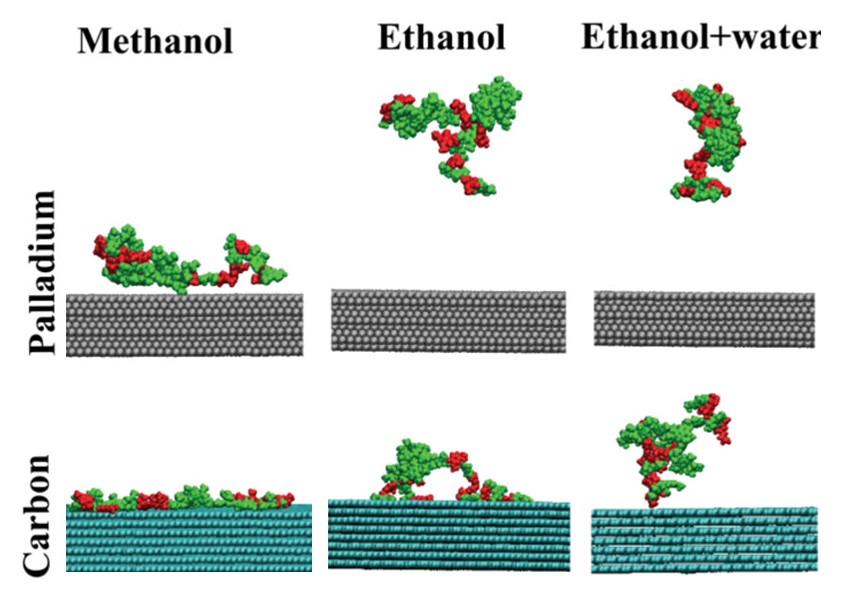
Here, scientists used computer modeling to simulate the movement of every atom in the system to better understand how lignin molecules behave in various organic solvents and how the dissolved lignin building blocks interact with catalyst surfaces. The results showed that some organic solvents are more effective than water at dissolving lignin, causing the molecules to spread out more and increasing the area accessible to interact with the catalyst. Additional modeling showed solvent selection affects how lignin molecules stick to the catalyst surface, a process called adsorption, which can affect the process of dissolving this polymer.
The Impact
Lignin is an abundant and renewable resource that could provide a domestic source of fuels and valuable chemicals. These findings show that modeling can provide molecular insights into the way that solvent choice influences catalytic reactions — a step towards optimizing lignin deconstruction — and highlight the need to consider both solvent effects and catalyst structure when designing RCF processes.
Summary
Scientists with the Great Lakes Bioenergy Research Center performed all-atom molecular dynamics simulations to study the solvation of lignin, solvent-mediated conformational changes, and the interaction of dissolved lignin oligomers, or short chains of repeating molecules, with model surfaces. They focused on the behavior of a model lignin oligomer in methanol, ethanol, a mixture of ethanol and water, and water alone at typical RCF reaction temperature (473 K) and room temperature. They also incorporated model palladium (Pd) and carbon (C) surfaces to understand how solvent choice impacts adsorption onto a representative catalytic surface to quantify competition between reactant and solvent molecules for the surface.
These simulations suggest strong adsorption of lignin on both Pd and C surfaces at 473 K with notable solvent-mediated differences in adsorption energies. Further thermodynamic calculations indicate that lignin adsorption is promoted by the entropy change resulting from displacement of solvent molecules from the surface.