UW–Madison researchers discovered that methylbutenol, a type of alcohol produced by the needles of some pine trees, outperformed two more common biofuels in reducing pollution and engine knock.
When it comes to making internal combustion engines run better, the solution may come from trees.
Scientists at the University of Wisconsin–Madison have discovered that when blended with gasoline methylbutenol, a type of alcohol produced by pine trees, produces less air pollution and is more effective than other alcohols at reducing engine knock, an annoying and sometimes destructive phenomenon that happens when fuel ignites when it’s not supposed to.
Together the findings suggest methylbutenol is a promising low-carbon biofuel that could be mixed with gasoline.
“Everything we’ve seen has been very, very favorable,” said David Rothamer, UW–Madison professor of mechanical engineering and coauthor of the studies published in the journals Combustion and Flame and Fuel.
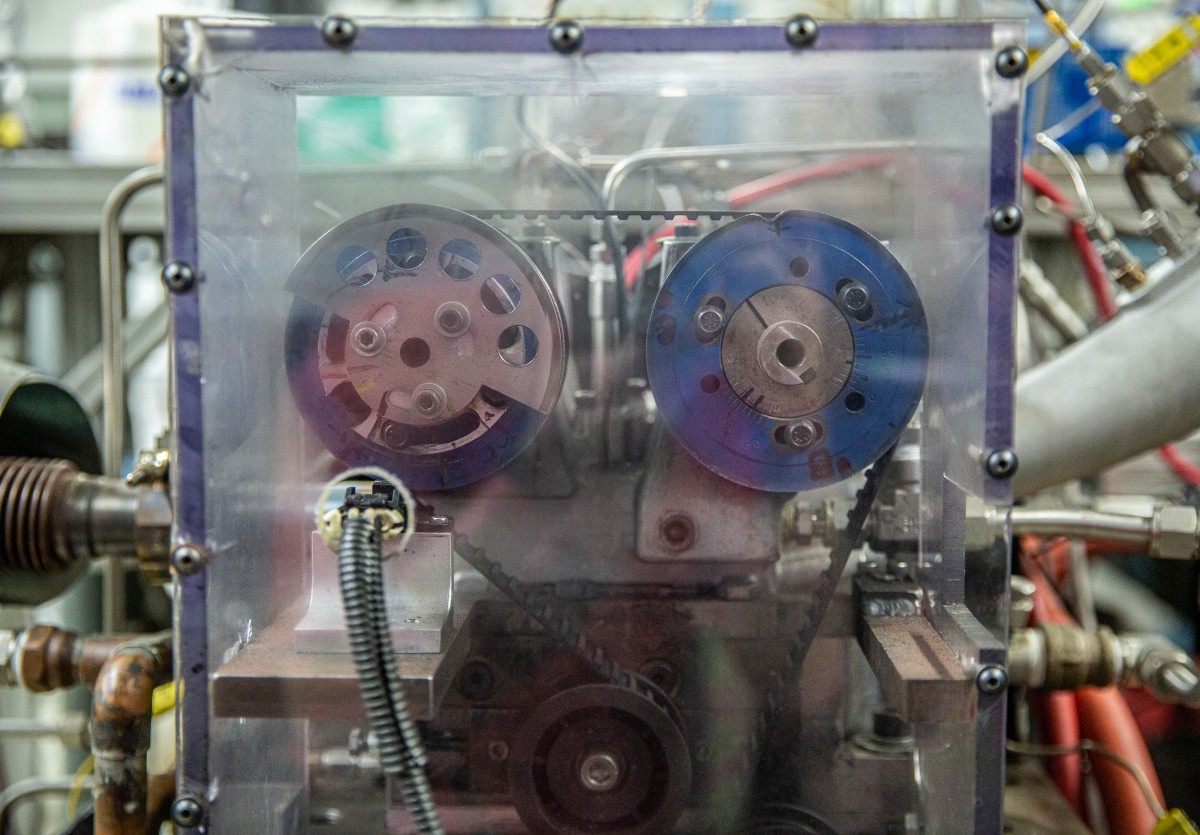
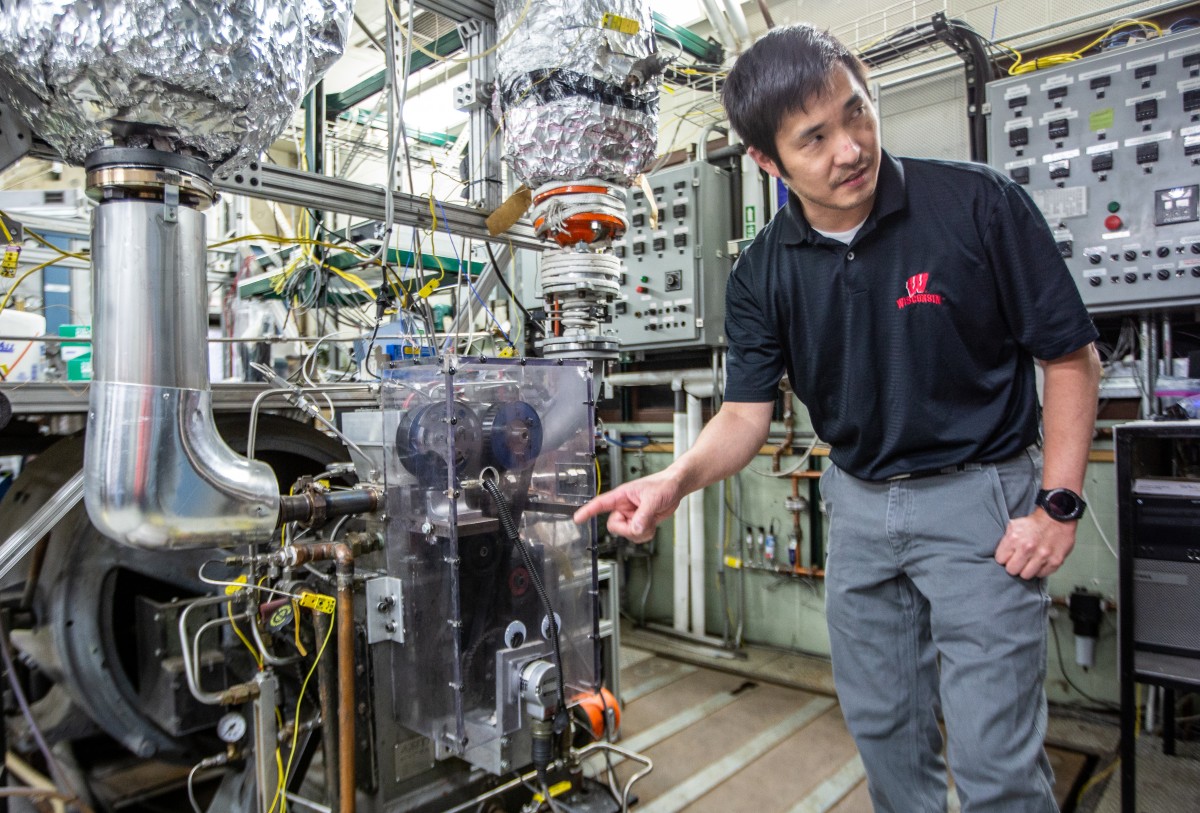
With support from the Great Lakes Bioenergy Research Center, a federally-funded research hub based at UW–Madison, Rothamer and his graduate student Stephen Sakai decided to investigate how three types of alcohol perform when mixed with gasoline in an internal combustion engine.
They compared ethanol with isobutanol and methylbutenol, which contain more carbon atoms, making them more similar to gasoline than ethanol.
With higher energy content and corrosion resistance, these next-generation biofuels are considered “drop-in” fuels that can replace gasoline without the need for modifying engines. GLBRC research is focused on eliminating barriers to cost-effective production of isobutanol and other chemicals from non-food plants like switchgrass and poplar.
Produced by many species of North American pines, methylbutenol is a type of terpenoid, a group of organic compounds that are a source of flavors, fragrances and medicines. Similar to isobutanol, it can also be produced using bioengineered microorganisms to ferment sugars in plant cells.
But unlike ethanol and isobutanol, methylbutenol has not been widely studied as a fuel additive.
“There hasn’t been much work done on these particular isomers, the C5 methylbutenol isomers,” Rothamer said. “At the time when we started there were just one or two papers written on them related to combustion behavior.”

Particulate matter, also known as soot, in vehicle exhaust is a major environmental and health concern.
Engine knock occurs when fuel explodes under high pressure, emitting pressure waves that cause wear and tear and in severe cases can destroy engine parts.
Knock is also one of the biggest barriers to increasing fuel efficiency because the higher compression needed to extract more energy from a drop of fuel can cause that fuel to ignite before it’s supposed to.
Most modern engines can automatically adjust the timing of the spark to eliminate knock, but this reduces efficiency, so refineries blend in additives to increase octane ratings, a measure of fuel’s ability to resist knock.
Historically refiners used tetraethyl lead, which spewed toxic fumes out the tailpipe, until the U.S. government began phasing out leaded gasoline in the 1970s. Today most gasoline is blended with ethanol, which boosts the octane rating but has some drawbacks: it contains less energy than gasoline and changes the fuel's properties in ways that can increase air pollution.

Rothamer and Sakai used a special single-cylinder engine in their lab on the UW–Madison campus to test each alcohol blended with gasoline at three levels based on the oxygen content.
For the first study, published in September, Rothamer and Sakai showed that increasing the amount of alcohol consistently reduced sooting and particulate emissions in each blend.
At the lowest blend levels, all three alcohols performed similarly. But at higher concentrations, methylbutenol produced the lowest level of soot, followed by isobutanol and ethanol.
That was surprising, Sakai said. “Based on its molecular composition you would expect it to soot more.”
Rothamer said the findings suggest there’s a molecular interaction that requires more investigation to understand.
In terms of knock suppression, ethanol and methylbutenol were slightly better than isobutanol at low blend levels. At higher levels, ethanol and isobutanol performed similarly, but methylbutenol did significantly better.
Rothamer said his findings, combined with recent Department of Energy research showing prenol can improve efficiency in turbocharged engines, indicate that terpene alcohols merit a closer look.
But don’t expect to fill your tank with methylbutenol anytime soon.
Sakai said much more research and analysis is needed to determine the lifecycle carbon footprint and whether it’s cost effective to produce methylbutenol as well as factors like toxicity, stability and material compatibility. The fuel would also need approval from the Environmental Protection Agency.
“It is promising as it stands now,” Sakai said.