The goal of replacing petroleum-based chemicals and fuels with plant-based products hinges on using as much of the plant matter as possible to keep costs and waste to a minimum.
We can look to nature for lessons on how to do this efficiently, says Gracielou Klinger, a chemistry and biochemistry graduate student at Michigan State University. “Bacteria and fungi break down plants through a number of different ways. When you see mushrooms growing on dead logs in a forest, they are breaking down the plant cell wall into small pieces that they ‘eat’ so that they can grow,” says Klinger, who works with Eric Hegg and James “Ned” Jackson at MSU.
In a new paper, Klinger and her colleagues in the Great Lakes Bioenergy Research Center (GLBRC) mimicked this natural approach to deconstructing lignin, an abundant but tough substance that makes up about a third of most plants. Their paper appears in a special issue of ChemSusChem focused on lignin valorization. Lignin’s complex molecular structure make it a potential source of an array of platform chemicals—molecular building blocks that can be used to make plastics, fabrics, pharmaceuticals and more. But that same complexity also makes lignin hard to break down, requiring harsh and expensive conditions. Here, Klinger describes the group’s efforts to find a more efficient and cost-effective way to turn the tough-to-manage substance into valuable molecules.
What was the focus of this study?
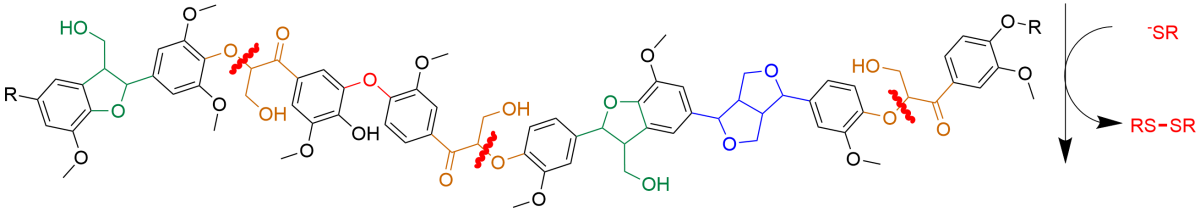
Because lignin is so complicated, we focused on an enzymatic process that cleaves the most common lignin bond, called beta-O-4. We chemically mimicked this process with thiols, which are simple organic compounds containing sulfur.
Previously, we published a proof-of-concept using this approach on simpler versions of lignin—small fragments like dimers and trimers, which have two or three lignin subunits linked together. In this newest publication, we moved on to polymer systems—larger, more complex lignin models that contain different kinds of chemical bonds. We found that our thiol-based process can do the same chemistries seen in the bacteria, not only on small lignin fragments but also on large lignin polymers.
Testing on small fragments lets us understand what is chemically happening to make sure we are mimicking the bacteria correctly. Then it was important to see if we could cleave bulky polymers that are more like real lignin. In future work, we are also hoping to increase the efficiency of our process by using electrochemistry to recycle the thiol compounds to decrease costs and increase yields.
How might this process be useful in industry?
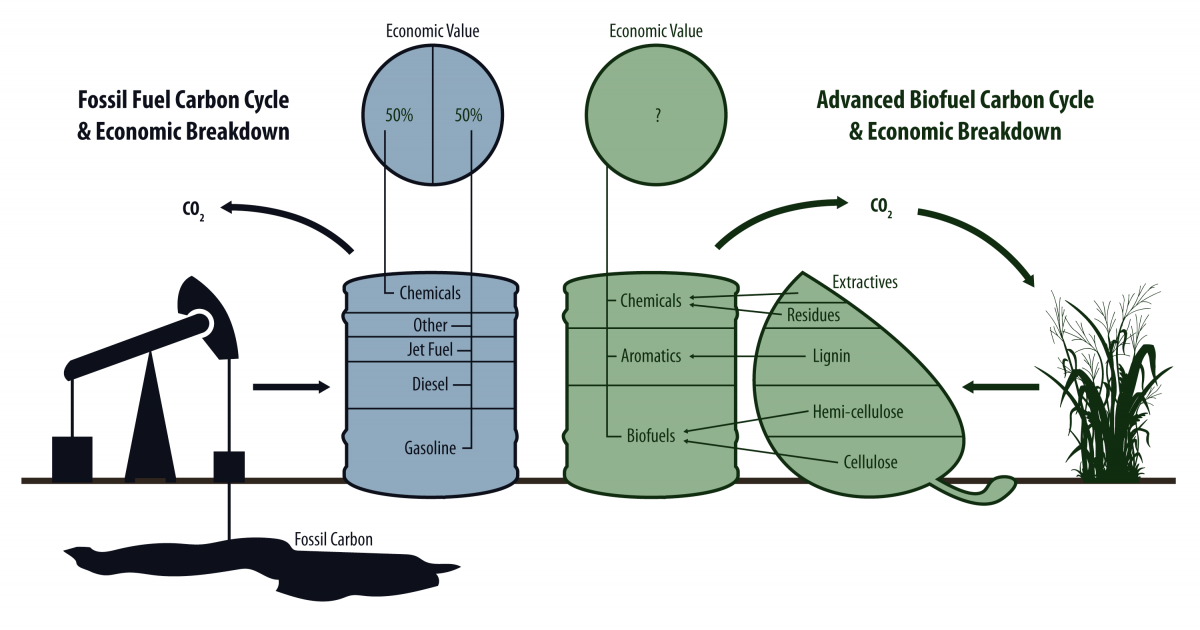
Many manufacturing processes can't deal effectively with large polymers, so breaking lignin into fragments, like monomers, dimers, trimers, etc., is important for moving toward industrial use. Then these smaller products can be converted into basically anything that fossil fuels can be converted into. Petroleum is distilled into various fractions for manufacturing, and the idea is that if fragmented lignin can be as well, we might be able to replace a petro-barrel with a bio-barrel.
What are the next steps for this research?
Well, they’re two-fold. First, we want to analyze the products we are forming and look at various industrial lignins to show that our process is “feedstock agnostic”—basically that it can work with any lignin. That’s important because lignin properties change based on how it is extracted from biomass. Second, we want to recycle our catalyst to allow for multiple "turn-overs" of our cleavage cycle. We envision this in an electrochemical cell. Right now we have focused our attention on this aspect of the project, which is key to keeping costs down.
This project included researchers at multiple GLBRC institutions. How was that important for the outcome?
If it weren't for the Center and the ease of passing samples back and forth and bouncing ideas off one another, the research would definitely not have progressed as fast as it has. The Donohue and Noguera labs at UW–Madison have done some beautiful work on the enzymatic process that we are mimicking. If it weren't for their work, we would not have a nice biological model to follow. The Ralph and Stahl labs, also at UW–Madison, lent their chemistry expertise, and the oxidation that the Stahl lab provided allowed us to really increase our yields.
Read more
Explore this topic further in our research highlight: https://www.glbrc.org/research/highlights/mimicking-wood-eaters-cleave-…
Reference
Klinger, G.E., Zhou, Y., Foote, J.A., Wester, A.M., Cui, Y., Alherech, M., Stahl, S.S., Jackson, J.E. and Hegg, E.L., “Nucleophilic Thiols Reductively Cleave Ether Linkages in Lignin Model Polymers and Lignin,” ChemSusChem (2020). [DOI: 10.1002/cssc.202001238]