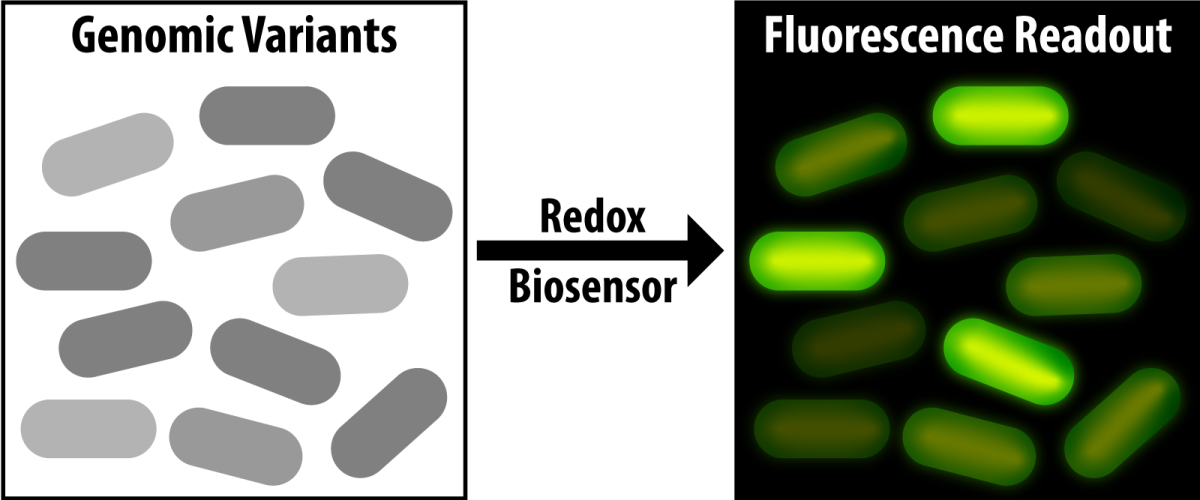
A major goal of the Great Lakes Bioenergy Research Center is to harness the power of microbes to create biofuels. But often, it’s an expensive challenge for scientists to identify the most useful individual variants among thousands of similar microbe strains. A new study led by Vatsan Raman, an assistant professor of biochemistry at the University of Wisconsin–Madison, unveils a biosensor that may light the way to the best microbial candidates for biofuel production.
You have to figure out a way of being able to sense the biofuel molecule inside the cell. That’s where this biosensor technology becomes really useful.
Vatsan Raman

In search of the best biofuel-producing microbes, scientists may need to make millions of variants via genetic modifications. But only a handful of these variants are likely to produce large amounts of biofuel. To identify the most promising strains, scientists currently have to crack open the cells and test for the desired molecules one microbe at a time, an expensive and extremely labor-intensive process.
In a paper published January 12, 2019, in ACS Synthetic Biology, Raman’s team describes a new biosensor capable of reporting a microbe’s biofuel production capacity to scientists from within the cell. It’s a faster, cheaper, and less disruptive means of identifying the most promising biofuel-producing microbes. The paper is co-authored by Yang Liu, a graduate student in Raman’s lab, and Robert Landick, UW–Madison professor of biochemistry and bacteriology. Liu is part of the Integrated Program in Biochemistry (IPiB), the joint graduate program of the Department of Biochemistry and Department of Biomolecular Chemistry.
“You have to figure out a way of being able to sense the biofuel molecule inside the cell,” Raman says. “So that’s where this biosensor technology becomes really useful.”
The biosensor protein designed by Raman’s team triggers green fluorescence in cells primed to make biofuel-type molecules. It responds to the ratio of two key cofactors, called NADH and NAD+, that are involved in shuttling electrons between molecules inside cells. Their ratio indicates what’s known as the redox state of a cell, which reveals the degree to which excess reducing equivalents are available for driving cellular reactions, such as making biofuels.
Cells maintain a balance between NADH and NAD+ during normal growth and respiration. But when excess NADH builds up in a bacterium, the cell can divert some of its energy away from routine growth and toward biofuel production.
Raman’s team specifically deleted the enzymes responsible for oxidizing NADH to NAD+ in variants of the bacterium E. coli, allowing NADH—and thus the cell’s biofuel-making potential—to build up. When the NADH:NAD+ ratio becomes abnormally high, the biosensor causes the cell to fluoresce. Highlighting these cells—literally—allows the scientists to isolate them for further experimentation.

"Our goal for the redox sensor is to identify redox changes in a high-throughput manner to speed up the discovery of mutants and conditions with elevated redox, which would be very labor-intensive using traditional analytical methods," says Yang Liu.
As a proof-of-concept for the biosensor, the team immersed cells engineered with high NADH among 10,000 wild-type E. coli cells. They were able to use the biosensor to detect the altered cells, showing its efficacy.
Raman says the biosensor has a variety of other potential applications in the field of biochemistry. Any time scientists are interested in learning about the redox state of a cell, it could come in handy. NADH is an important metabolic marker, and imbalances of NADH/NAD+ can be a sign of a variety of diseases in humans, including cancer. This means observing differences in redox state could have a host of implications in medicine and biotechnology in the future.
“What we think we have is a really simple tool that folks in the lab can use to measure the redox state of their cell type of interest,” Raman says.