Platinum is a highly reactive and in-demand catalyst across the chemical and energy industries, but a team of University of Wisconsin-Madison and Georgia Institute of Technology scientists could reduce the world’s dependence on this scarce and expensive metal.
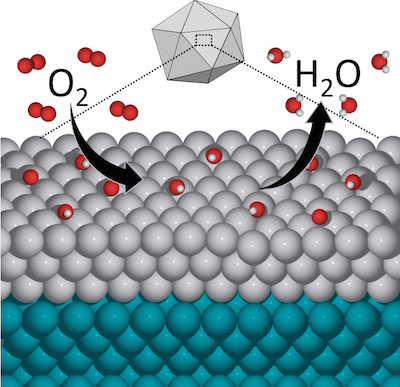
In a paper published July 2 in the journal Nature Communications, Professor Manos Mavrikakis and his research group describe a new catalyst that combines platinum with the less expensive metal palladium. This not only reduces the need for platinum but actually proves significantly more catalytically active than pure platinum in the oxygen reduction reaction, a chemical process key to fuel cell energy applications. The palladium-platinum combination also proves more durable, compounding the advantage of getting more reactivity with less material. Just as importantly, the paper offers a way forward for chemical engineers to design still more new catalysts for a broad range of applications by fine-tuning materials on the atomic scale.





